Abstract
The treatment paradigm for acute myeloid leukemia (AML) includes regimens each specified for a target population. Unfit patients (pts) and those of older age are often limited in their treatments considering the toxicity profile associated with traditional induction regimens. Venetoclax (Ven), a potent BCL-2 inhibitor, is frequently administered for older adults who are deemed ineligible for more intensive chemotherapies. However, its clinical use remains a challenge due to its severe myelosuppressive effects. Despite the generally accepted clinical practice of delaying the next cycle or reducing the total Ven dosage per cycle, there is minimal data evaluating the effectiveness and resource needs under these circumstances. The objective of our analysis was to review the clinical course and identify survival rates in our pts undergoing treatment with Ven for AML.
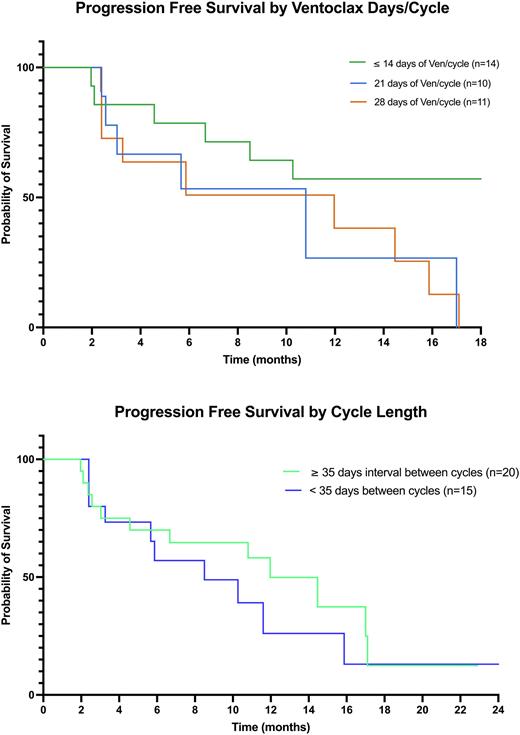
We retrospectively reviewed the clinical and molecular data of newly diagnosed and relapsing/refractory AML pts admitted to North Shore University Hospital for induction treatment with a hypomethylating agent (HMA) plus Ven from Nov 2019 to May 2022. Pts were categorized into subgroups based on their median number of days on Ven per cycle excluding induction cycle (≤ 14 days, 21 days, 28 days) as well as the median number of interval days between cycles (< 35 days or ≥ 35 days). Pts who did not complete 2 cycles were excluded from subgroup analyses. Median progression-free survival (mPFS) was defined as the time from Ven initiation to disease progression or death from any cause. PFS was analyzed with the Kaplan-Meier (KM) method and log rank testing. One-way analysis of variance and t-test was used to compare clinical interventions in each subgroup.
A total of 53 pts underwent induction therapy with Ven + HMA: 79.2% frontline and 20.1% relapsed/refractory. The median age was 76 years-old (range 60-91). Of these 53 pts, 62.2% (n=33) were de novo, 24.5% (n=13) had AML with myelodysplastic related changes and 13.2% had therapy related AML (n=7). Utilizing the 2022 ELN risk categories (Dohner et al, Blood 2022), < 1% (n=3) were favorable risk while 33.9% (n=18) were intermediate risk and 60.3% (n=32) were adverse risk. All prognostic indicators were evenly dispersed among the subgroups. 33.9% of pts (n=18) died within their induction treatment admission. The mPFS was not reached in the subgroup of pts receiving ≤ 14 days of Ven/cycle while it was 10.8 months in pts receiving 21 days of Ven/cycle and 11.9 months for pts receiving 28 days of Ven/cycle. Although there was no significant difference between KM estimates when comparing number of days on Ven/cycle, there was a trend favoring ≤14 days of Ven/cycle. Patients who had ≥ 35 days interval between cycles had a mPFS of 11.9 months versus 8.5 months in pts who had < 35 days intervals. However, their KM estimates were not significantly different (p > 0.05). Pts included in subgroup analysis, received an average of 6.7 cycles and required an average of 2.6 hospital admissions while on a Ven regimen. Pts received an average of 4.8 pRBC units and 6.1 platelet units per cycle. Although there was a trend favoring reduction in Ven dosing (≤ 14 day subgroup), there was no significant difference in number of cycles, number of hospital admissions and transfusion requirements between each subgroup analysis (p > 0.05).
This retrospective analysis demonstrates no difference in Ven outcomes with dose de-escalation and increased interval time between cycles in pts requiring such adjustments. The mPFS of each subgroup analyses was also similar compared to the mPFS (14.7 months) reported in the phase 3 clinical trial leading to its FDA approval (DiNardo et al, NEJM 2020; NCT02993523). Interestingly, the subgroup with a median dosing of ≤ 14 days of Ven/cycle had yet to reach a mPFS at 18 months. Also, the review of our patients’ clinical courses progress demonstrated a trend in decreased transfusion requirements and hospitalizations with lower Ven dosing although not statistically significant. Our analysis suggests that further larger retrospective and prospective enquiries are needed to evaluate the potential benefit of Ven dose de-escalation not only in terms of PFS and treatment related complications, but also for quality of life.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal